Introduction to Units and Measurements is an important topic from JEE Main / IIT JEE Advanced Exam Point of view. Some questions can be asked directly. Most importantly, the whole Physics includes this topic. Thus, it is very important to have a clear cut on this topic. This study notes on Unit, Dimensions and Measurement will help you in revising the topic before the JEE Main & IIT JEE Advanced Exam.
You can also download Introduction to Units and Measurements notes in the PDF format at the end of this article.
- Dimensional Formula:-
Dimensional formula of a physical quantity is the formula which tells us how and which of the fundamental units have been used for the measurement of that quantity.
- How to write dimensions of physical quantities:-
(a) Write the formula for that quantity, with the quantity on L.H.S. of the equation.
(b) Convert all the quantities on R.H.S. into the fundamental quantities mass, length and time.
(c) Substitute M, L and T for mass, length and time respectively.
(d) Collect terms of M,L and T and find their resultant powers (a,b,c) which give the dimensions of the quantity in mass, length and time respectively.
[embeddoc url=”https://aimstutorial.in/wp-content/uploads/2020/10/Unit-and-measurment.pdf” download=”all” viewer=”google”]
- Characteristics of Dimensions:-
(a) Dimensions of a physical quantity are independent of the system of units.
(b) Quantities having similar dimensions can be added to or subtracted from each other.
(c) Dimensions of a physical quantity can be obtained from its units and vice-versa.
(d) Two different physical quantities may have same dimensions.
(e) Multiplication/division of dimensions of two physical quantities (may be same or different) results in production of dimensions of a third quantity.
Unit, Dimensions, and Measurement Notes
[embeddoc url=”https://aimstutorial.in/wp-content/uploads/2020/10/2.UNITS-AND-MEASUREMENTS-4-37.pdf” download=”all” viewer=”google”]
- To measure or express a physical quantity we need a standard of measurement so that different measurements of one physical quantity can be related with respect to each other. This standard is called the unit of the specific physical quantity.
- To measure any physical quantity, we need two parts = Numerical value (n) × Unit (u)
- Numerical value gives how many times the physical quantity is measured with respect to the standard unit. The second part gives the name of the unit.
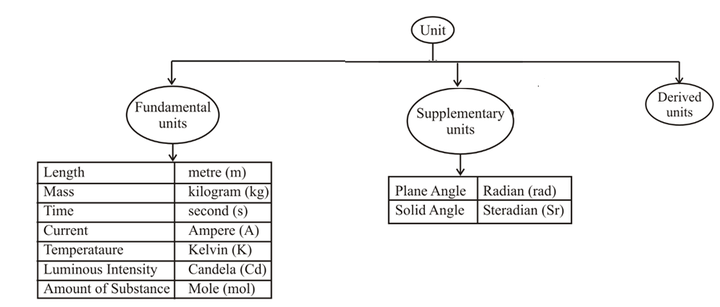
- Base Units: The units for the fundamental or base quantities are called fundamental or base units.
- Derived Units: The units of all other physical quantities can be expressed as combinations of the base units. Such units obtained for the derived quantities are called derived units.
1. The International System of Units
Following are a few measurement systems that are used.
- CGS System- This system of the unit is based on the centimetre as the unit of length, gram as the unit of the mass, and second as the unit of the time.
- FPS System- This system of the unit is based on the foot as the unit of length, pound as the unit of the mass, and second as the unit of the time.
- MKS System- This system of the unit is based on the meter as the unit of length, kilogram as the unit of the mass, and second as the unit of the time.
The system of units which is at present internationally accepted for measurement is the (SI System – International System of Units). It is based on the MKS system.
SI Quantity and Units
2. Accuracy, Precision, and Errors in Measurement
- Accuracy – The accuracy of a measurement is a measure of how close the measured value is to the true value of the quantity. It depends on the number of significant figures in it. The larger the significant digit the higher the accuracy.
- Precision- Precision is the degree of exactness. It depends on the least count of measuring instrument. The smaller the least count, the more precise will be measurement.
e.g. Suppose the exact (true) value of a certain mass is 45.2646 kg. Let it measure by an instrument as 45.2 by an instrument of least count 0.1 and 45.17 by other instruments of least count 0.01.
The first reading is more accurate because it is closer to the true value but less precise because its resolution is 0.1. The second reading is more precise because its resolution is 0.01 but less accurate.
- Error
The uncertainty in a measurement is called error. Every calculated quantity which is based on measured values also has an error.
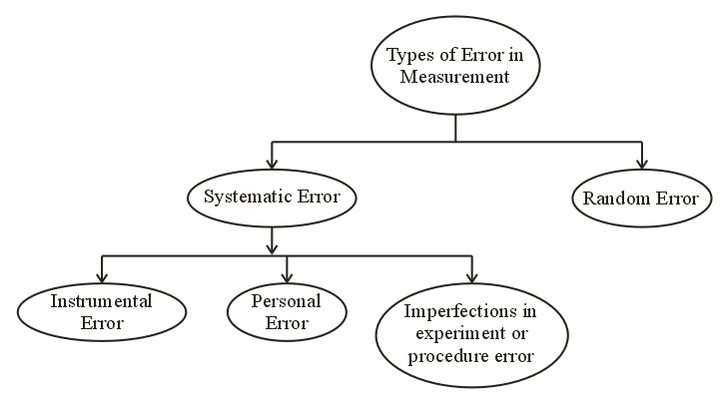
Error in measurement can be broadly classified as (1) Systematic error and (2) Random error.
- Systematic Error: Systematic error is caused due to the fault of the measuring device, design of the experiment, or imperfect method of observation. These errors can be reduced by improving experimental conditions, repeating measurement using a different method or different equipment.
- Random Error: The random error is those errors, which occur due to random and unpredictable fluctuations in experimental conditions personal error by the observer. These errors can be reduced by conducting repeat trials, using precise apparatus.
Error in measuring the value of a physical quantity can be expressed by
- Absolute error: The magnitude of the difference between the measured and the true value of the quantity is called the absolute error of the measurement.
e.g. let us assume that true value or exact of a physical quantity is ATrue and the measured value is Amea., then
the absolute error in Physical quantity A is = | ATrue – Amea.|
- Relative error: The ratio of absolute error to the true value in measuring a physical quantity is known as relative error.
e.g. let us assume that true value or exact of a physical quantity is ATrue and the measured value is Amea., then
the relative error in Physical quantity A is =
- Percentage error: When the relative error is expressed in percent, it is called the percentage error.
e.g. let us assume that true value or exact of a physical quantity is ATrue and the measured value is Amea., then
the percentage error in Physical quantity A is =
Rules of arithmetic operation for error
- In case of Addition and Subtraction
Suppose a physical quantity(Z) depends on the other quantity A and B, Z = A ± B
Error in quantity A is ΔA and in B is ΔB then for both addition and subtraction the absolute error is added up.
Absolute error in Z is ΔZ = ΔA + ΔB, And
Percentage error in the value of Z is
- In case of Multiplication and Divisions
Suppose a physical quantity(Z) depends on the other quantity A and B such a way that
Z = AB or
Then for both multiplication and division, the percentage error or relative are added up. Then
Relative error in the value of Z is
Percentage error in the value of Z is
- In the case of the Power function
Suppose a physical quantity(Z) depends on the other quantity A, C, and B such a way that
In the condition of measuring error, the power of each physical quantity multiplied by respectively. Then
Relative error in value of Z is
Percentage error in value of Z is
3. Significant Figures
Significant figures indicate the precision of measurement which depends on the least count of the measuring instrument.
Rules of Significant figures
- All the non-zero digit are significant.
- All the zeros between two non-zero digit are significant, no matter where the decimal point is.
- If the number is less than 1, the zeroes on the right of decimal point but to the left of the first non-zero digit are non-significant. e.g. in 0.00532, zero before digit 5 is non-significant
- The terminal zeros in a number without a decimal point are not significant.
e.g. 45200 cm = 452 m has three significant figures.
- The trailing zeros in a number with a decimal point are significant. e.g. 54.500 has five significant figures.
Rules for Arithmetic Operations with Significant Figures
- In case of Addition and subtraction
In addition or subtraction, the result should retain as many decimal places as are there in the number with the least decimal places.
e.g. 656.34 m + 73.2463 m + 624.14 m = 1353.726 m, therefore the result be rounded off to 1353.73 m
- In case of Multiplication and Division
In multiplication or division, the result should retain as many decimal places as are there in the original number with the least significant figures.r4e
e.g. Force = 13.55 kg × 12.563 m/s2 = 170.22865 N, therefore the result be rounded off to 170.23 N
Rounding off the Uncertain Digits
- Preceding digit is raised by 1 if it the insignificant digit to be dropped is more than 5.
e.g. 55.686 is rounded off to 55.69
- Preceding digit is unchanged if it the insignificant digit is less than 5.
e.g. 55.681 is rounded off to 55.68
- If the insignificant digit is 5 and the preceding digit is even, the insignificant digit is dropped and if it is odd, the preceding digit is raised by 1.
e.g. 55.685 is rounded off to 55.68
e.g. 55.675 is rounded off to 55.68
4. Dimension of Physical Quantities
The dimension of a physical quantity can be defined as the powers to which the base quantities are raised to represent that quantity. All the physical quantities can be expressed in terms of base quantities.
Let’s take an example:
Consider a physical quantity – density
So, the dimension of density is 1 in mass, and -3 in Length. Thus,
Dimensional formula of the density = [ML-3]
5. Dimensional Analysis and its Applications
- To convert a physical quantity from one system of unit to another
For any system of unit, Numerical value(n)×unit (u) = constant. So, on changing unit, numerical value will also get changed.
Let n1u1 is the value of the physical quantity in one system of unit and n2u2 is the value in other systems then,
Using the above method, we can convert a physical quantity from one system of unit to another.
- To check the dimensional correctness of physical equation.
It is based on the principle of homogeneity, which states that a given physical equation is dimensionally correct if the dimension of the various terms on either side of the equation is the same. But if the dimension of either side of the equation is not same then the physical equation is wrong.
e.g. Let us consider an equation, , where m is mass, is distance, g is gravity, and t is the time.
The dimension of LHS and RHS are
[M][L2] = [M][LT-2][T]
[ML2] = [MLT-1]
Since both side dimension unequal, so the given equation is dimensionally incorrect.
- To establish a relationship between different physical quantities
If we know the dependency of the physical quantity on other quantities, then we can find the relation among different quantity by using the principle of homogeneity.
e.g. Let us consider a physical quantity time t, it depends on length , mass m, and gravity g. Then we can find the relation of time among other quantity
let time depends on length, mass, and gravity as the power of a, b, and c respectively. Then
Equating the dimension of both sides
Substituting the value of a, b, and c in equation (1)
Introduction to Units and Dimensions
Every measurement has two parts. The first is a number (n) and the next is a unit (u). Q = nu. For Example, the length of an object = 40 cm. The number expressing the magnitude of a physical quantity is inversely proportional to the unit selected.
If n1 and n2 are the numerical values of a physical quantity corresponding to the units u1 and u2, then n1u1 = n2u2. For Example, 2.8 m = 280 cm; 6.2 kg = 6200 g.
Fundamental and Derived Quantities(Introduction to Units and Dimensions)
- The quantities that are independent of other quantities are called fundamental quantities. The units that are used to measure these fundamental quantities are called fundamental units. There are four systems of units namely C.G.S, M.K.S, F.P.S, and SI.
- The quantities that are derived using the fundamental quantities are called derived quantities. The units that are used to measure these derived quantities are called derived units.
Fundamental and supplementary physical quantities in SI system:
| Fundamental
Quantity |
System of units | ||
| C.G.S. | M.K.S. | F.P.S. | |
| Length | centimeter | Meter | foot |
| Mass | gram | Kilogram | pound |
| Time | second | Second | second |
| Physical quantity | Unit | Symbol |
| Length | Meter | m |
| Mass | kilogram | kg |
| Time | second | s |
| Electric current | ampere | A |
| Thermodynamic temperature | kelvin | K |
| Intensity of light | candela | cd |
| Quantity of substance | mole | mol |
Supplementary Quantities:
| Plane angle | radian | rad |
| Solid angle | steradian | sr |
Most SI units are used in scientific research. SI is a coherent system of units.
A coherent system of units is one in which the units of derived quantities are obtained as multiples or submultiples of certain basic units. SI system is a comprehensive, coherent and rationalized M.K.S. Ampere system (RMKSA system) and was devised by Prof. Giorgi.
- Meter: A meter is equal to 1650763.73 times the wavelength of the light emitted in vacuum due to electronic transition from 2p10 state to 5d5 state in Krypton-86. But in 1983, 17th General Assembly of weights and measures adopted a new definition for the meter in terms of velocity of light. According to this definition, a meter is defined as the distance traveled by light in vacuum during a time interval of 1/299, 792, 458 of a second.
- Kilogram: The mass of a cylinder of platinum-iridium alloy kept in the International Bureau of weights and measures preserved at Serves near Paris is called one kilogram.
- Second: The duration of 9192631770 periods of the radiation corresponding to the transition between the two hyperfine levels of the ground state of cesium-133 atoms is called one second.
- Ampere: The current which when flowing in each of two parallel conductors of infinite length and negligible cross-section and placed one meter apart in vacuum, causes each conductor to experience a force of 2 × 10-7 newtons per meter of length is known as one ampere.
- Kelvin: The fraction of 1/273.16 of the thermodynamic temperature of the triple point of water is called Kelvin.
- Candela: The luminous intensity in the perpendicular direction of a surface of a black body of area 1/600000 m2 at the temperature of solidifying platinum under a pressure of
101325 Nm-2 is known as one candela. - Mole: The amount of a substance of a system which contains as many elementary entities as there are atoms in 12 × 10-3 kg of carbon-12 is known as one mole.
- Radian: The angle made by an arc of the circle equivalent to its radius at the center is known as radian. 1 radian = 57o17l45ll.
- Steradian: The angle subtended at the center by one square meter area of the surface of a sphere of radius one meter is known as steradian.
Some Important Conclusions Introduction to Units and Dimensions
- Angstrom is the unit of length used to measure the wavelength of light. 1 Å = 10-10 m.
- Fermi is the unit of length used to measure nuclear distances. 1 Fermi = 10-15 meter.
- A light year is the unit of length for measuring astronomical distances.
- Light year = distance traveled by light in 1 year = 9.4605 × 1015 m.
- Astronomical unit = Mean distance between the sun and earth = 1.5 × 1011 m.
- Parsec = 3.26 light years = 3.084×1016 m
- Barn is the unit of area for measuring scattering cross-section of collisions. 1 barn = 10-28 m2.
- Chronometer and metronome are time measuring instruments. The quantity having the same unit in all the systems of units is time.
⇒ Also Read: List of all SI Units
| MACRO Prefixes | MICRO Prefixes |
| Kilo (K) 103
Mega (M) 106 Giga (G) 109 Tera (T) 1012 Peta (P) 1015 Exa (E) 1018 Zetta (Z) 1021 Yotta (y) 1024 |
Milli (m) 10-3
(μ) 10-6 nano (n) 10-9 pico (p) 10-12 femto (f)10-15 atto (a) 10-18 zepto (z) 10-21 yocto (y) 10-24 |
Note: The following are not used in the SI system.
- deca 101 deci 10-1
- hecta 102 centi 10-2
How to write Units of Physical Quantities?
Full names of the units, even when they are named after a scientist should not be written with a capital letter. Eg: Newton, watt, ampere, meter.
- The unit should be written either in full or in agreed symbols only.
- Units do not take the plural form. Eg: 10 kg but not 10 kgs, 20 w but not 20 ws.
- No full stop or punctuation mark should be used within or at the end of symbols for units. Eg: 10 W but not 10 W.
What are Dimensions?
Dimensions of a physical quantity are the powers to which the fundamental units are raised to obtain one unit of that quantity.
Dimensional Analysis
Dimensional analysis is the practice of checking relations between physical quantities by identifying the dimensions of the physical quantities. These dimensions are independent of the numerical multiples and constants and all the quantities in the world can be expressed as a function of the fundamental dimensions.
Read More: Dimensional Analysis
Dimensional Formula
The expression showing the powers to which the fundamental units are to be raised to obtain one unit of a derived quantity is called the dimensional formula of that quantity.
If Q is the unit of a derived quantity represented by Q = MaLbTc, then MaLbTc is called dimensional formula and the exponents a, b and, c are called the dimensions.
What are Dimensional Constants?
The physical quantities which have dimensions and have a fixed value are called dimensional constants. e.g.: Gravitational constant (G), Planck’s constant (h), Universal gas constant (R), Velocity of light in a vacuum (C), etc.
What are the Dimensionless quantities?
Dimensionless quantities are those which do not have dimensions but have a fixed value.
- Dimensionless quantities without units: Pure numbers, π, e, sin θ, cos θ, tan θ etc.
- Dimensionless quantities with units: Angular displacement – radian, Joule’s constant – joule/calorie, etc.
What are Dimensional variables?
Dimensional variables are those physical quantities which have dimensions and do not have a fixed value. e.g.: velocity, acceleration, force, work, power, etc.
What are the Dimensionless variables?
Dimensionless variables are those physical quantities which do not have dimensions and do not have a fixed value. For example Specific gravity, refractive index, the coefficient of friction, Poisson’s ratio, etc.
Law of Homogeneity of Dimensions
- In any correct equation representing the relation between physical quantities, the dimensions of all the terms must be the same on both sides. Terms separated by ‘+’ or ‘–’ must have the same dimensions.
- A physical quantity Q has dimensions a, b and c in length (L), mass (M) and time (T), respectively and n1 is its numerical value in a system in which the fundamental units are L1, M1 and T1 and n2 is the numerical value in another system in which the fundamental units are L2, M2 and T2, respectively then
Limitations of Dimensional Analysis
- Dimensionless quantities cannot be determined by this method. Constant of proportionality cannot be determined by this method. They can be found either by experiment (or) by theory.
- This method is not applicable to trigonometric, logarithmic and exponential functions.
- In the case of physical quantities which are dependent upon more than three physical quantities, this method will be difficult.
- In some cases, the constant of proportionality also possesses dimensions. In such cases, we cannot use this system.
- If one side of the equation contains addition or subtraction of physical quantities, we cannot use this method to derive the expression.
Some Important Conversions
- 1 bar = 106 dyne/cm2 = 105 Nm-2 = 105 pascal
- 76 cm of Hg = 1.013×106 dyne/cm2 = 1.013×105 pascal = 1.013 bar.
- 1 toricelli or torr = 1 mm of Hg = 1.333×103 dyne/cm2 = 1.333 millibar.
- 1 kmph = 5/18 ms-1
- 1 dyne = 10-5 N,
- 1 H.P = 746 watt
- 1 kilowatt hour = 36×105 J
- 1 kgwt = g newton
- 1 calorie = 4.2 joule
- 1 electron volt = 1.602×10-19 joule
- 1 erg = 10-7 joule
Some Important Physical Constants
- Velocity of light in vacuum (c) = 3 × 108 ms-1
- Velocity of sound in air at STP = 331 ms-1
- Acceleration due to gravity (g) = 9.81 ms-2
- Avogadro number (N) = 6.023 × 1023/mol
- Density of water at 4oC = 1000 kgm-3 or 1 g/cc.
- Absolute zero = -273.15oC or 0 K
- Atomic mass unit = 1.66 × 10-27 kg
- Quantum of charge (e) = 1.602 × 10-19 C
- Stefan’s constant = 5.67 × 10–8 W/m2/K4
- Boltzmann’s constant (K) = 1.381 × 10-23 JK-1
- One atmosphere = 76 cm Hg = 1.013 × 105 Pa
- Mechanical equivalent of heat (J) = 4.186 J/cal
- Planck’s constant (h) = 6.626 × 10-34 Js
- Universal gas constant (R) = 8.314 J/mol–K
- Permeability of free space () = 4π × 10-7 Hm-1
- Permittivity of free space () = 8.854 × 10-12 Fm-1
- The density of air at S.T.P. = 1.293 kg m-3
- Universal gravitational constant = 6.67 × 10-11 Nm2kg-2
Derived SI units with Special Names:
| Physical quantity | SI unit | Symbol |
| Frequency | hertz | Hz |
| Energy | joule | J |
| Force | newton | N |
| Power | watt | W |
| Pressure | pascal | Pa |
| Electric charge or
quantity of electricity |
coulomb | C |
| Electric potential difference and emf | volt | V |
| Electric resistance | ohm | |
| Electric conductance | siemen | S |
| Electric capacitance | farad | F |
| Magnetic flux | weber | Wb |
| Inductance | henry | H |
| Magnetic flux density | tesla | T |
| Illumination | lux | Lx |
| Luminous flux | lumen | Lm |
Dimensional Formulas for Physical Quantities
| Physical quantity | Unit | Dimensional formula |
| Acceleration or acceleration due to gravity | ms–2 | LT–2 |
| Angle (arc/radius) | rad | MoLoTo |
| Angular displacement | rad | MoloTo |
| Angular frequency (angular displacement/time) | rads–1 | T–1 |
| Angular impulse (torque x time) | Nms | ML2T–1 |
| Angular momentum (Iω) | kgm2s–1 | ML2T–1 |
| Angular velocity (angle/time) | rads–1 | T–1 |
| Area (length x breadth) | m2 | L2 |
| Boltzmann’s constant | JK–1 | ML2T–2θ–1 |
| Bulk modulus (.) | Nm–2, Pa | M1L–1T–2 |
| Calorific value | Jkg–1 | L2T–2 |
| Coefficient of linear or areal or volume expansion | oC–1 or K–1 | θ–1 |
| Coefficient of surface tension (force/length) | Nm–1 or Jm–2 | MT–2 |
| Coefficient of thermal conductivity | Wm–1K–1 | MLT–3θ–1 |
| Coefficient of viscosity (F =) | poise | ML–1T–1 |
| Compressibility (1/bulk modulus) | Pa–1, m2N–2 | M–1LT2 |
| Density (mass / volume) | kgm–3 | ML–3 |
| Displacement, wavelength, focal length | m | L |
| Electric capacitance (charge/potential) | CV–1, farad | M–1L–2T4I2 |
| Electric conductance (1/resistance) | Ohm–1 or mho or siemen | M–1L–2T3I2 |
| Electric conductivity (1/resistivity) | siemen/metre or Sm–1 | M–1L–3T3I2 |
| Electric charge or quantity of electric charge (current x time) | coulomb | IT |
| Electric current | ampere | I |
| Electric dipole moment (charge x distance) | Cm | LTI |
| Electric field strength or Intensity of electric field (force/charge) | NC–1, Vm–1 | MLT–3I–1 |
| Electric resistance () | ohm | ML2T–3I–2 |
| Emf (or) electric potential (work/charge) | volt | ML2T–3I–1 |
| Energy (capacity to do work) | joule | ML2T–2 |
| Energy density () | Jm–3 | ML–1T–2 |
| Entropy () | Jθ–1 | ML2T–2θ–1 |
| Force (mass x acceleration) | newton (N) | MLT–2 |
| Force constant or spring constant (force/extension) | Nm–1 | MT–2 |
| Frequency (1/period) | Hz | T–1 |
| Gravitational potential (work/mass) | Jkg–1 | L2T–2 |
| Heat (energy) | J or calorie | ML2T–2 |
| Illumination (Illuminance) | lux (lumen/metre2) | MT–3 |
| Impulse (force x time) | Ns or kgms–1 | MLT–1 |
| Inductance (L) (energy =) or
coefficient of self-induction |
henry (H) | ML2T–2I–2 |
| Intensity of gravitational field (F/m) | Nkg–1 | L1T–2 |
| Intensity of magnetization (I) | Am–1 | L–1I |
| Joule’s constant or mechanical equivalent of heat | Jcal–1 | MoLoTo |
| Latent heat (Q = mL) | Jkg–1 | MoL2T–2 |
| Linear density (mass per unit length) | kgm–1 | ML–1 |
| Luminous flux | lumen or (Js–1) | ML2T–3 |
| Magnetic dipole moment | Am2 | L2I |
| Magnetic flux (magnetic induction x area) | weber (Wb) | ML2T–2I–1 |
| Magnetic induction (F = Bil) | NI–1m–1 or T | MT–2I–1 |
| Magnetic pole strength (unit: ampere–meter) | Am | LI |
| Modulus of elasticity (stress/strain) | Nm–2, Pa | ML–1T–2 |
| Moment of inertia (mass x radius2) | kgm2 | ML2 |
| Momentum (mass x velocity) | kgms–1 | MLT–1 |
| Permeability of free space () | Hm–1 or NA–2 | MLT–2I–2 |
| Permittivity of free space (.) | Fm–1 or C2N–1m–2 | M–1L–3T4I2 |
| Planck’s constant (energy/frequency) | Js | ML2T–1 |
| Poisson’s ratio (lateral strain/longitudinal strain) | –– | MoLoTo |
| Power (work/time) | Js–1 or watt (W) | ML2T–3 |
| Pressure (force/area) | Nm–2 or Pa | ML–1T–2 |
| Pressure coefficient or volume coefficient | oC–1 or θ–1 | θ–1 |
| Pressure head | m | MoLTo |
| Radioactivity | disintegrations per second | MoLoT–1 |
| Ratio of specific heats | –– | MoLoTo |
| Refractive index | –– | MoLoTo |
| Resistivity or specific resistance | –m | ML3T–3I–2 |
| Specific conductance or conductivity (1/specific resistance) | siemen/metre or Sm–1 | M–1L–3T3I2 |
| Specific entropy (1/entropy) | KJ–1 | M–1L–2T2θ |
| Specific gravity (density of the substance/density of water) | –– | MoLoTo |
| Specific heat (Q = mst) | Jkg–1θ–1 | MoL2T–2θ–1 |
| Specific volume (1/density) | m3kg–1 | M–1L3 |
| Speed (distance/time) | ms–1 | LT–1 |
| Stefan’s constant. | Wm–2θ–4 | MLoT–3θ–4 |
| Strain (change in dimension/original dimension) | –– | MoLoTo |
| Stress (restoring force/area) | Nm–2 or Pa | ML–1T–2 |
| Surface energy density (energy/area) | Jm–2 | MT–2 |
| Temperature | oC or θ | MoLoToθ |
| Temperature gradient () | oCm–1 or θm–1 | MoL–1Toθ |
| Thermal capacity (mass x specific heat) | Jθ–1 | ML2T–2θ–1 |
| Time period | second | T |
| Torque or moment of force (force x distance) | Nm | ML2T–2 |
| Universal gas constant (work/temperature) | Jmol–1θ–1 | ML2T–2θ–1 |
| Universal gravitational constant (F = G. ) | Nm2kg–2 | M–1L3T–2 |
| Velocity (displacement/time) | ms–1 | LT–1 |
| Velocity gradient (dv/dx) | s–1 | T–1 |
| Volume (length x breadth x height) | m3 | L3 |
| Water equivalent | kg | MLoTo |
| Work (force x displacement) | J | ML2T–2 |
Quantities Having the Same Dimensional Formula
- Impulse and momentum.
- Work, energy, torque, the moment of force, energy.
- Angular momentum, Planck’s constant, rotational impulse.
- Stress, pressure, modulus of elasticity, energy density.
- Force constant, surface tension, surface energy.
- Angular velocity, frequency, velocity gradient.
- Gravitational potential, latent heat.
- Thermal capacity, entropy, universal gas constant and Boltzmann’s constant.
- Force, thrust.
- Power, luminous flux.
Applications of Dimensional Analysis
Dimensional analysis is very important when dealing with physical quantities. In this section, we will learn about some applications of the dimensional analysis.
Fourier laid down the foundations of dimensional analysis. The Dimensional formulas are used to:
- Verify the correctness of a physical equation.
- Derive a relationship between physical quantities.
- Converting the units of a physical quantity from one system to another system.
Checking the Dimensional Consistency
As we know, only similar physical quantities can be added or subtracted, thus two quantities having different dimensions cannot be added together. For example, we cannot add mass and force or electric potential and resistance.
For any given equation, the principle of homogeneity of dimensions is used to check the correctness and consistency of the equation. The dimensions of each component on either side of the sign of equality are checked, and if they are not the same, the equation is considered wrong.
Let us consider the equation given below,
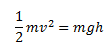
The dimensions of the LHS and the RHS are calculated
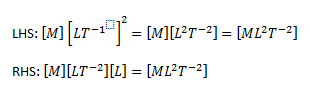
As we can see the dimensions of the LHS and the RHS are the same, hence, the equation is consistent.
Deducing the Relation among Physical Quantities
Dimensional analysis is also used to deduce the relation between two or more physical quantities. If we know the degree of dependence of a physical quantity on another, that is the degree to which one quantity changes with the change in another, we can use the principle of consistency of two expressions to find the equation relating these two quantities. This can be understood more easily through the following illustration.
Example: Derive the formula for centripetal force F acting on a particle moving in a uniform circle.
As we know, the centripetal force acting on a particle moving in a uniform circle depends on its mass m, velocity v and the radius r of the circle. Hence, we can write
Hence,
F = ma vb rc
Writing the dimensions of these quantities,
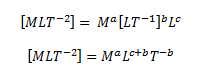
As per the principle of homogeneity, we can write,
a = 1, b + c = 1 and b = 2
Solving the above three equations we get, a = 1, b = 2 and c = -1.
Hence, the centripetal force F can be represented as,
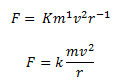
Frequently Asked Questions On Dimension Analysis
What is the meaning of dimension in physics?
It is an expression that relates derived quantity to fundamental quantities. But it is not related to the magnitude of the derived quantity.
What is the dimension of force?
We know, F = ma —– (1)
Mass is a fundamental quantity but acceleration is a derived quantity and can be represented in terms of fundamental quantities.
a = [LT−2] —– (2)
Using (1) and (2),
F = [MLT−2]
This is the dimension of force.
What is dimensional analysis?
Dimensional analysis is based on the principle that two quantities having the same dimensions can only be compared with one another. For example, I can compare kinetic energy with potential energy and say they equal or one is greater than another because they have the same dimension. But I cannot compare kinetic energy with force or acceleration as their dimensions are not the same.